Does oil oxidation create trans fats?

I am considering switching to beef tallow for my high heat cooking, as it has a high smoke point and is a saturated fat (which means, that it does not oxidize easily). Also recent randomized control trials suggest, that saturated fat on its own is not causally linked to heart disease problems.
From what I have learned, is that unsaturated fats, even those with high smoke points, oxidize easily, because of their chemical property of being unsaturated, which means, that they have "space" for other electrons and react easily. From the unsaturated fats, the polyunsaturated fats have twice as much potential for reaction and oxidization as the monounsaturated fats, because they have two "arms".
With beef tallow for example, it seems to be the other way around: It has a high smoke point, but even more important: It's a saturated fat and it doesn't make new bonds easily, because it has all its electrons.
Now, my question is, how are trans fats being created? Are they the product of oxidized polyunsaturated oils? Where do trans fats come from? Or are companies intentionally putting them in food for economic reasons?
I am aware, that trans fats are banned in the US, but they are not banned in EU yet.
Best Answer
First, a little chemistry primer on what unsaturated fatty acids look like (this is petroselinic acid):
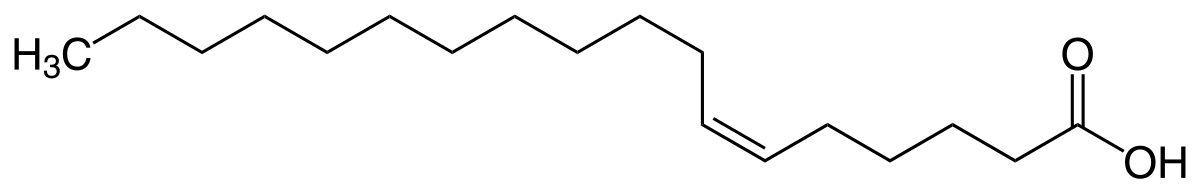
By Yikrazuul (talk) - Own work, Public domain, Link
You can see the double bond near the "middle" of the molecule. The "rest" of the molecule is attached to the same side of the double bond axis on both ends, making this a "cis" fatty acid. Rotation around double bonds requires a relatively high amount of activation energy, so this "cis" configuration is stable, at least at low-ish temperatures.
An example of a "trans" fatty acid would be elaidic acid:
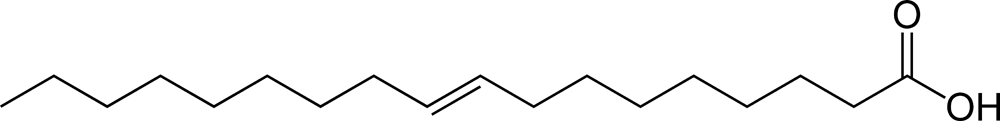
By Benjah-bmm27 - Own work, Public domain, Link
Here you can see that the molecule continues on opposite sides of the double bond axis ("trans"). At higher (e.g. frying pan) temperatures, unsaturated fatty acids more readily isomerize between cis and trans, and since trans is energetically favorable, more unsaturated fatty acids will be in their trans configuration after being heated to a high temperature.
So to answer the question(s): No, trans fatty acids are not generated by oxidation, but the same conditions that favor oxidation (high temperature) also favor the generation of trans fats from cis fats. Also, the bacteria in the digestive tracts of ruminants (cattle, sheep, but also deer etc.) produce a significant proportion of trans fatty acids. In addition, the simplest (and cheapest) industrial processes to saturate ("harden") unsaturated fats produce a relatively high amount of trans fats. So yes, not adopting other processes can be economical, if this can be considered "intentional"...your call.
A more extensive explanation can also be found in the accepted answer to this question: Does preparation of food change the nutritional content with respect to fat type?
Pictures about "Does oil oxidation create trans fats?"



Quick Answer about "Does oil oxidation create trans fats?"
High temperature treatment can cause the formation of trans fat in oils through oxidation process.Does oil turn into trans fat?
Cooking oils do not hydrogenate or create trans fats during home cooking, even beyond the smoke point. A 1999 study published in the International Journal of Fats and Oils fried potatoes in olive oil at 356\xb0F for 15 minutes. The oil was reused 8 times and sampled after each use.What happens when oil is oxidized?
Oil oxidation is an undesirable series of chemical reactions involving oxygen that degrades the quality of an oil. Oxidation eventually produces rancidity in oil, with accompanying off flavours and smells. All oil is in a state of oxidation - you cannot stop it completely - but there are ways to reduce it.What creates trans fat?
Most of the trans fat in the foods we eat is formed through a manufacturing process that adds hydrogen to vegetable oil, which converts the liquid into a solid fat at room temperature. This process is called hydrogenation.What oil causes trans fat?
Most trans fats are formed through an industrial process that adds hydrogen to vegetable oil, which causes the oil to become solid at room temperature. This partially hydrogenated oil is inexpensive and less likely to spoil, so foods made with it have a longer shelf life.Unsaturated vs Saturated vs Trans Fats, Animation
Sources: Stack Exchange - This article follows the attribution requirements of Stack Exchange and is licensed under CC BY-SA 3.0.
Images: Anna Shvets, Ono Kosuki, Andrea Piacquadio, Maxi Gagliano
