What does "bring to a simmer" mean?

First, a confession: I work in software, so I'm probably paying way too much attention to the state of liquid that is "a simmer". That written, I love to cook, and no recipe direction gives me more confusion, sadness, and googling than "bring to a simmer". Accept no substitute. I find this to be the most vague direction in all of culinary science, and it drives what's left of my organized mind insane.
So here's the setting. I'm making vichyssoise, because I'm intrigued by the possibility of making a dish that has no color at all. I've been instructed to "bring to a boil and simmer the soup for 35 minutes."
The internet is filled with unsatisfying and at times contradictory answers. My research yields a few prototypical examples:
- "Simmer" means "low or off position," suggesting basically no heat at all.
- To "simmer" is to heat to a temperature point just off boiling, generally acknowledged as somewhere around 95 degrees C or something like 195 degrees F.
- "Simmer" is something like a "soft boil," a vague state that appears to be between "not bubbling" and "roiling", but which by definition must boil in some way, since you know, it's bubbling.
Each of these examples mean fundamentally different things. As far as I can tell, a "simmer" is a phase transition whereby the suspension in question, whatever the soup, sauce, or solid (apparently you "simmer" bratwurst, you never boil it) may be, cooks in a way that only years of experience or training can identify. Hence, my question:
What does "simmer" mean? Does it differ per recipe or is it universally defined?
EDIT: Did a bad copy/paste job from another window.
Best Answer
Personally, I would argue that 2 and 3 are actually the same, and they are your answer.
If you heat a pan of water you'll notice the bubbles forming before the water is actually boiling, hence the talk of between not bubbling and full on roiling.
Also, when you're making your soup, it isn't pure water, so the boiling temp will not be a perfect 100 degrees C in any case.
So, I would say, that simmering is when you keep it just under a full boil. Watch what you're cooking, there should be gentle movement, but not a full roiling pan of whatever it is you're cooking.
To get something simmering away, you need to bring up to a full boil, then reduce the heat until you're getting movement, but not full bubbling.
Pictures about "What does "bring to a simmer" mean?"



Simmer vs. Boil
More answers regarding what does "bring to a simmer" mean?
Answer 2
Colloquially, simmer means to maintain a liquid at a temperature where relatively few, small vapor bubbles form, while boil means to maintain a liquid at a temperature where relatively many, large vapor bubbles form.
If the liquid is being stirred, the temperature of the liquid will be at its boiling point (100°C for distilled water, depending on atmospheric conditions) regardless of whether it is simmering or boiling. If the liquid is not being stirred, a liquid that appears to be simmering may have reached its boiling point near the heat source, causing vapor bubbles to form, but may not have reached its boiling point distal to the heat source. Thus the average temperature of the liquid may be below the boiling point.
Practically, food in a liquid that is simmering will be cooked at the same temperature or near the same temperature as food in a liquid that is boiling. Adding more heat to a liquid at its boiling point will not increase the liquid's temperature, but will increase the rate of vaporization, and hence the number and size of bubbles (at an extreme, detonating an atomic bomb next to your stove would cause the liquid (among other things) to essentially instantaneously vaporize). This leads to two differences in the cooking methods:
- A boiling liquid will reduce at a faster rate than a simmering liquid. If you are trying to reduce the liquid's volume, boiling may be preferred. If not, simmering may be preferred.
- The larger and more numerous bubbles of a boiling liquid can physically harm delicate food items. Delicate noodles and vegetables may be more damaged in a boiling liquid than in a simmering liquid. Potatoes are less prone to such damage.
Returning to the three potential definitions that your research found:
- "Simmer means low or off position" - This statement is false, but is derived from common labeling on stove ranges. On some ranges, the temperature dials will be labelled 'simmer' at their lowest setting. This should be thought of as 'maintain simmer'. The idea is that after a liquid is brought to an obvious boil, the temperature is at its boiling point. By turning the heat off, energy is lost as the liquid vaporizes and escapes into the atmosphere, causing the temperature of the liquid to gradually fall below its boiling point. By maintaining a minimal amount of heat at the lowest 'simmer' setting, the energy lost through vaporization can be replaced, maintaining the liquid at its boiling point. In my experience, this tends to work well for liquids in a pot with a small surface area. However, for liquids in pots with a large surface area, significant heat is also lost through radiation, and I'll have to turn the dial up to the '1' or '2' setting to maintain the boiling point.
- "To simmer is to heat to a temperature point just off boiling, generally acknowledged as somewhere around 95 degrees C or something like 195 degrees F." - This statement is generally true. As noted above, if vapor bubbles are forming in a liquid, at least part of it is at its boiling point. So, it is possible for the bottom of a pot of water to reach 100°C while the top of the pot is only 90°C, and perhaps the average temperature of the entire pot of water is around 95°C. Stirring the water distributes the heat evenly, and a simmering, stirred pot of water will be uniformly at 100°C.
- "'Simmer' is something like a 'soft boil', a vague state that appears to be between 'not bubbling' and 'roiling', but which by definition must boil in some way, since you know, it's bubbling." - I think this is mostly in line with how I have described the difference between simmering and boiling above.
I would casually define the two terms as follows:
- To simmer is to add the minimal amount of energy to maintain a liquid at its boiling point, resulting in few, relatively small vapor bubbles.
- To boil is to add additional energy to a liquid that is already at its boiling point, resulting in many, relatively large vapor bubbles.
To learn about the science behind cooking, I recommend Harold McGee's On Food and Cooking: The Science and Lore of the Kitchen.
Answer 3
It is very simple. A simmer is when the liquid at the very of the bottom of pan boils, but not all the liquid. You get little bubbles, no roil.
Answer 4
I would go with this answer from America's Test Kitchen. (A decent thermometer helps.)
The difference between simmering and boiling water can mean the difference between a chunky vegetable soup and a bowl of mush. Water reaches its boiling point and starts to evaporate at 212 degrees F, while a simmer is generally between 185 and 205 degrees. If bubbles aggressively break the surface of the water, it's boiling; if the bubbles are smaller and gentler, it’s simmering.
Answer 5
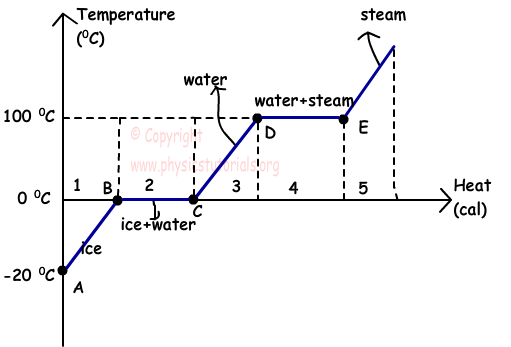
Take a look at a phase transition diagram for water:
The important idea contained in the chart is that in order to get water (or any material, really) to switch from one phase to another (e.g. liquid to gas, or gas to liquid) you have to add or remove energy. If you have a beaker of pure water at exactly 100°C, the liquid water doesn't all just explode into water vapor at the same time -- it takes the addition of extra energy just to switch from liquid to gas.
The difference between a simmer and a hard boil is the rate at which the liquid is changing phase from liquid to gas. If you have a pot of liquid at the boiling point (whatever that temperature is for the liquid in question) and you add more heat quickly, such as with a burner set to the highest setting, you'll get lots of bubbles and a "hard boil" because the liquid is changing phase rapidly. If you add heat slowly, as a burner set to low does, then you get just a few bubbles because the liquid changes phase slowly and that's what simmering is. The temperature of the liquid is the same in both cases, it's just the rate of phase change that's different.
Answer 6
I think that the term is usually used like, eg "bring to a boil then simmer for 5 minutes" would be, when you boil something you would normally do it on high heat...but after the ingredients are put in the pot, depending on the food you would sometimes keep the fire burning strong "boil", or "simmer" it at low heat. So the food still cooks but the liquid does not evaporate as speedily. So if you are following a recipe where it says to simmer for 10 minutes, but you keep the pot a full boil, then a lot of the liquid would evaporate resulting in a different then "recipe ideal" flavor, consistency turn out.
Answer 7
Simmering at 195-205 degrees f. Or 95-97 degrees c. That's at sealevel. Increase the temp. by one degree f. for every 1000 ft below sealevel or decrease one degree f. for every 1000 ft. above sealevel. Thats what I've done for 70 years and it works.
Sources: Stack Exchange - This article follows the attribution requirements of Stack Exchange and is licensed under CC BY-SA 3.0.
Images: Brett Jordan, Brett Jordan, Brett Jordan, Brett Jordan